Bronschhofen - Switzerland
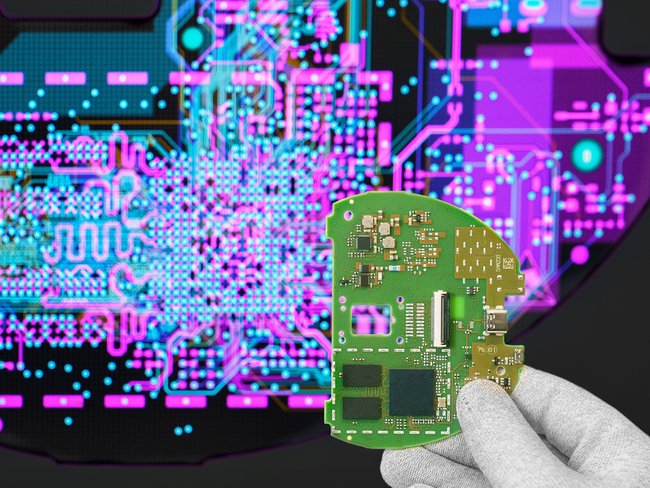
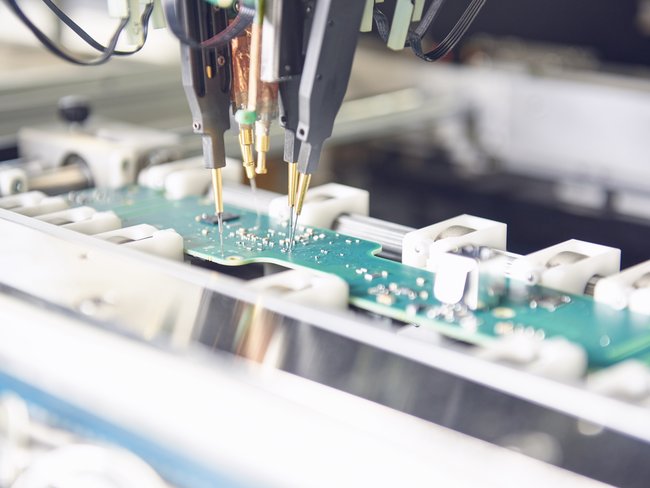
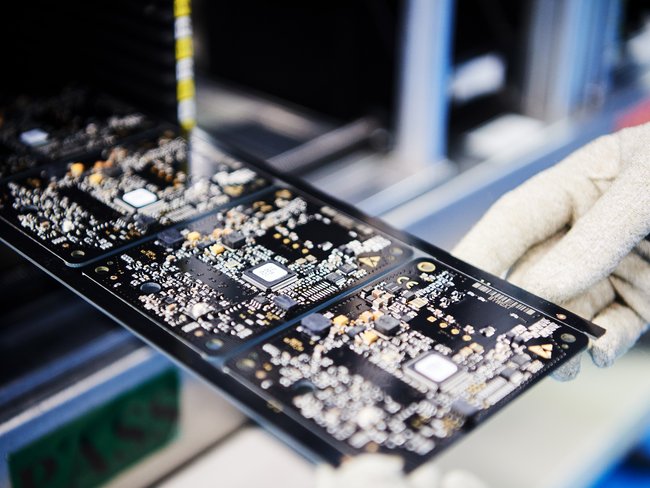
The Cicor location in Bronschhofen offers complete outsourcing solutions for the development and production of electronic assemblies as well as complete devices and systems. Cicor develops and produces small and medium sized series of electronic assemblies and systems using state-of-the-art equipment at its Bronschhofen site. The location has extensive expertise in medical technology. In the newly created, protected assembly area of ISO class 8, medical devices with increased demands on the assembly environment are manufactured. The EMS site in Bronschhofen has a diverse customer portfolio from the medical, industrial, consumer, defence and transport markets.
Solutions
Contact
Swisstronics Contract Manufacturing AG
Gebenloostrasse 15
9552 Bronschhofen
Switzerland